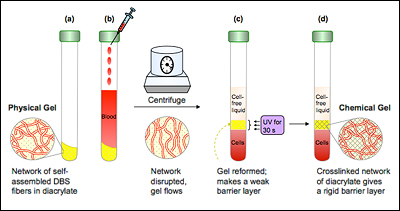
News Story
"Smart" Fluid Controlled with Light
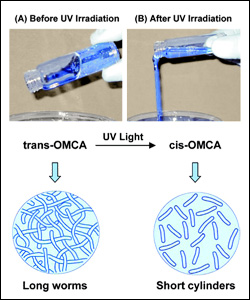
Photoresponsive (PR) fluids consisting of CTAB and OMCA. When OMCA is in its trans from, its mixture with CTAB gives rise to long, entangled wormlike micelles. Upon UV irradiation, trans-OMCA gets photoisomerized to cis-OMCA, and the corresponding change in molecular geometry causes a drastic reduction in micellar length.
Fluids capable of a state-change in response to electric fields (electrorheological, ER), magnetic fields (magnetoreheological, MR), and light (photorheological, PR) change instantly from liquids into semi-solids or gels, then back again as the stimulus is applied or removed. ER- and MR-based smart fluids are already in wide use in devices such as shock absorbers for vehicles and buildings, valves and clutches, but their light-triggered cousins have so far remained largely ignored by industry because they are typically difficult and expensive to manufacture. The Raghavan group, however, has engineered new kinds of PR fluids that can be created using inexpensive chemicals found in most labs. The researchers believe that their discovery could spur on the acceptance and use of PR fluids in commercial applications, particularly in microscale devices such as microvalves or microsensors, where light will be more effective than electrical or magnetic fields for precision control.
The group’s smart fluids contain micelles, strings of molecules that spontaneously form in water under the right conditions. Initially, the micelles are long, wormlike chains that tend to get entangled, much like a bowl of spaghetti, making the fluid thick and gel-like. Upon exposure to UV radiation, the micelles’ length is drastically reduced due to rearrangements of their constitutent molecules. The shortened micelles become untangled, and the smart fluid becomes a thin, water-like liquid, with a viscosity (thickness) that is reduced by 4 orders of magnitude (a factor of 10,000).
The Complex Fluids and Nanomaterials Group’s work on this project was funded by a seed grant from the Small Smart Systems Center (SSSC) at the University of Maryland and a CAREER award from the NSF-CTS.
Learn More:
Visit the Complex Fluids and Nanomaterials Group website »
ACS Publications' article citation »
Read the materials@nature article »
Read the ScienceDaily article »
Read the Chemical Processing article »
Download the Materials Today article (PDF) »
Published March 16, 2007